Using an On-Farm Culturing System to Identify Mastitis Causing Pathogens
Using an On-Farm Culturing System to Identify Mastitis Causing Pathogens
Mastitis causes substantial economic losses from reduced production, treatment costs, discarded milk, lost bonuses, and increased labor. Bacteriological milk culture results can help producers make educated treatment decisions. Additionally, understanding mastitis at the herd level can help producers make informed decisions on prevention and treatment strategies for the future. However, the biggest challenge with culturing has been the time it takes to receive results back from the lab. Now, on-farm culture systems enable producers to run bacteriological cultures in just 24 hours on their own farm for less cost than sending samples to off-farm laboratories. The cost of supplies for on-farm cultures runs about $3 per sample plus the initial incubator purchase ($50 to 100).
Benefits
When milk from clinical mastitis cases is cultured before treatment, antibiotic treatment can be selected for cases that are likely to benefit from antibiotic therapy, reducing antibiotic use by as much as 50%. This system enables you to identify Gram-positive infections (like Strep. dysgalactiae, Strep. uberis, or coagulase negative staph.) which often respond to antibiotics, and Gram-negative infections (like E. coli or Klebsiella), which should not be treated with intramammary antibiotics unless the mastitis becomes toxic.
On-farm culturing enables producers to obtain bacteriological results in just 24 hours. Because many mastitis cases are not cured by antibiotics, withholding antibiotic treatment for 24 hours does not really affect treatment success rates. Cows that need treatment (those with Gram-positive infections) can be treated once the results are obtained. Cows with cases that will not respond to antibiotics (those with Gram-negative infections) may be monitored to ensure that they are systemically treated if the immune system is unable to fight the infection and the mastitis becomes toxic. However, the cows that fight off the Gram-negative infections successfully will not have been treated with antibiotics, meaning no treatment costs and no milk discard.
Implementation strategies
The possibilities of how to use on-farm culture systems are immense. Probably the most valuable group to sample is clinical mastitis cases so that educated treatment decisions can be made. Another valuable practice is to sample all quarters of your highest somatic cell count cows, based on your DHI results each month. Fresh cows are another important group to target. The fresh period, or the period from the day of calving to 21 days in milk, is a time when cows are most susceptible to mastitis. Mastitis in the fresh period has an effect on the entire lactation. Managing mastitis in these cows can minimize its detrimental effects. The other most susceptible period of mastitis occurrence is during the dry period. While most dairy advisors recommend blanket dry cow treatment, or treating all cows with a dry cow antibiotic at dry-off, selective dry cow treatment is another option. Sampling cows the day before dry off would enable producers to decide whether to dry cow treat a cow or not. While this would reduce antibiotic use and cost, being infection-free at dry-off does not mean that the cow will be infection-free at calving or throughout her lactation.
How to get started
Materials needed for sample collection include:
- Sterile culturing tubes (your milk co-operative may supply these for free or reduced cost)
- Cotton swabs soaked with 70% ethyl alcohol for disinfecting teats (storing these in a small re-sealable food storage container works well as long as it is kept moist with alcohol and kept sealed when not in use)
- Nitrile gloves
- A permanent marker for labeling
- A cooler with ice or a designated refrigerator
Materials needed for culturing include:
- A small incubator (set at 98.6°F; an egg incubator is a low-cost option, ranging from $50 to 100)
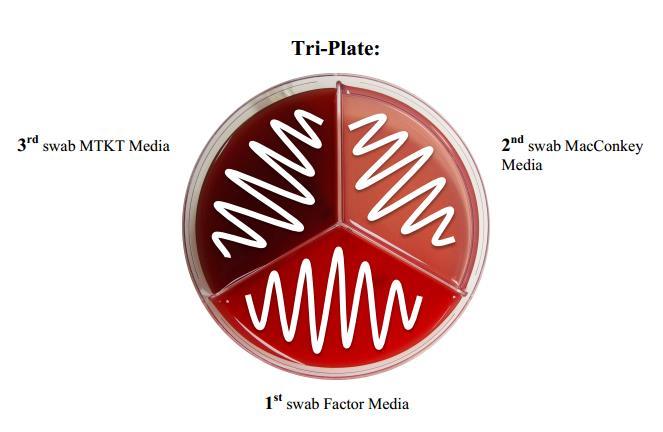
- Tri-plates (with modified TKT agar, Factor agar, and MacConkey agar; keep refrigerated until use)
- Nitrile gloves
- Sterile cotton swabs
- A permanent marker for labeling
- A designated waste bin for discarded plates and swabs (plates with bacterial growth are considered a biohazard so you will need to work with your veterinarian to find a proper disposal method)
Your on-farm laboratory does not need to be large or extravagant, but does need to be in a clean, designated area with an uncluttered table or countertop. The table or countertop should be made of a material that is easy to disinfect in-between each use. The room should hold a relatively constant humidity (75% works best) – parlors and washrooms are not recommended because of the high moisture level.
First, aseptic culture milk samples should be taken from the desired quarters. Samples can be plated immediately, but can also be refrigerated or kept in a cooler with ice for up to two days. Storing samples in a refrigerator used to store human food or drinks is not recommended because of the risk of bacterial contamination and then consumption. Once in the lab, the sample should be gently mixed by holding the sample between a finger and thumb then rotating back and forth a few times. Next, a sterile cotton swab should be placed inside the tube with care taken to avoid touching the cotton part of the swab to anything except the milk and the agars. The swab should be streaked in a zigzag motion within each plate division onto the Factor, MacConkey, and then modified TKT, as illustrated in the photo below, placing the same swab back into the sample in-between each zigzag. Some agar may be absorbed onto the cotton swab when touched and may prevent growth of certain pathogens so it is important to streak in the order described. Next, plates should be flipped upside down (so that the lid is on the bottom) and placed in the incubator for 24 hours. Results can then be compared to the pathogen identification pictures included in the Minnesota On-Farm Culturing System User Guide.
Throughout all steps in this process, care should be taken to not touch the plate media or inside of the milk sample tube with anything except the cotton swab. Nitrile gloves should be worn throughout the sampling and plating process. The table or countertop should be disinfected with a household cleaner (like Lysol® disinfecting wipes) or bleach (1 part chlorine bleach mixed with 9 parts water) before each plating (to help prevent plate contamination) and after each plating (to prevent human contact with the pathogens). Many mastitis-causing pathogens can also cause human illness (like E. coli and Staphylococcus aureus) through ingestion or through the bloodstream via wounds. Always clean your work areas and wash your hands after lab work, even though gloves are worn.
Who should culture
Ideally, the same person treating the cows will also take and plate the milk samples, allowing that person to monitor each cow’s progress and apply both a microbiological and cow management mentality to each case. Records of cow identification, culture result, treatment, withholding and withdrawal times, and treatment efficacy (if treated, did the antibiotic work?) should always be kept. Records can help determine what treatments work best and help determine your specific herd’s bacterial prevalence (is there a high proportion of infections being caused by a certain type of pathogen?) and preventive methods can be taken against the prevalent pathogens.
Collecting samples from individual quarters is recommended as opposed to composite samples (comingled milk from 2 or more quarters) because interpretation of results is difficult. For example, if one quarter is infected with a Gram-positive pathogen and another quarter is infected with a Gram-negative pathogen, it is impossible to tell which quarter contains the Gram-positive pathogen in order to treat only that quarter.
Potential sources of frustration for beginners
If milk samples are not obtained aseptically, they may contain contaminants, or bacteria from the teats, udder, or milkers hands that are not responsible for the actual mastitis infection. Contaminants may cause confusion on pathogen identification because the growth on the plate may no longer match the identification pictures. More than two different pathogens will not likely survive in the same udder quarter because of resource limitations and competition so a sample is also considered contaminated if more than two different pathogens are present in a sample. When contaminants grow on the agar, differentiation of mastitis-causing pathogens from the contaminants is extremely difficult and the cow must be re-sampled. It is more common for this to occur when learning the sampling technique and with first lactation and fresh cows, which may kick or move around when being sampled. Practicing sampling techniques is the only way to overcome this obstacle. Once the method is perfected, contaminated samples should occur in less than 5% samples.
Some plates may result in no bacterial growth, even if a cow has obvious signs of mastitis. This occurs for several reasons. First, little to no bacteria may be left in the udder, a result of the cow’s immune system combating the infection before milk sampling occurred. Keep in mind that the clots, flakes, and abnormal milk secretions are a result of udder damage from the bacterial assault, not from the bacteria themselves. It takes some time for the udder to heal and the cow may show clinical signs for days after the infection is cured, even if the infection was short-lived. No growths can also occur if the sample was not shaken well enough before being plated onto the agar. Bacteria are not evenly distributed throughout the milk culture so it is important to shake the sample a bit to disperse them. The last source of no growths is that the bacteria were not shedding, or being expelled from the body, at the time that the sample was taken. This is sometimes a difficult obstacle to overcome and the only way to detect these pathogens is continuous sampling to catch the pathogen when it is in a shedding state.
Mycoplasma requires special agar to grow and thus will not grow on any of the three agars in the tri-plate. Mycoplasma is usually not included in lab cultures either so if you or your veterinarian suspects a Mycoplasma infection, consider requesting this test at your local laboratory.
Conclusions
On-farm culturing is less expensive than sending cultures to a laboratory and has the potential to decrease mastitis costs and antibiotic use in your herd. It is also quick and relatively simple to obtain similar results to your local laboratory. Using on-farm culturing results to determine treatment decisions has the potential to decrease mastitis costs, antibiotic use, and discarded milk, and improve animal welfare.
Authors: Amanda Sterrett and Jeffrey Bewley
